Generate high-quality therapeutic leads for GPCR targets in just 12 weeks with next-generation antibody discovery workflows
The therapeutic promise of GPCR-targeted monoclonal antibodies
G protein-coupled receptors (GPCRs) represent the largest family of cell surface receptors, serving as critical signaling nodes across all major physiological systems. These seven-transmembrane proteins control essential functions in metabolism, immunity, and neurotransmission, making them invaluable drug targets. GPCRs operate through a sophisticated mechanism of action involving ligand binding, conformational change, followed by downstream signaling activation of a diverse range of pathways. Leveraging this mechanism, GPCR-targeting monoclonal antibodies enable precise targeting of specific GPCR conformations and signaling pathways, offering advantages over small molecules including improved selectivity and diverse modes of action.1
Despite the therapeutic advantages and physiological importance, a striking clinical gap exists. Currently, over 35% of all marketed drugs target GPCRs, yet a small fraction of the approximately 800 GPCRs encoded in the human genome have been successfully targeted with antibody therapeutics, amounting to only four FDA-approved GPCR-targeted antibodies.2 This disparity highlights both the challenge and the opportunity in developing antibody therapeutics against these crucial targets.
Challenges in GPCR-targeted monoclonal antibody discovery
The unique structural features of GPCRs create significant hurdles for antibody discovery. As multi-pass transmembrane proteins, GPCRs require lipid bilayer stabilization to maintain their native conformation. They are notoriously difficult to solubilize and purify without disrupting function, making it challenging to present them in their native form for immunization or screening. And perhaps most critically, many GPCRs have limited extracellular domains available for antibody recognition, severely restricting epitope accessibility.
Traditional antibody discovery methods face numerous obstacles when applied to GPCRs. Wild-type mice often have limited immune responses to human GPCRs due to high sequence homology between species. Hybridoma technology, while widely used, suffers from low throughput and limited diversity, typically capturing only a tiny fraction of the immune repertoire. This approach often misses rare but potentially valuable clones and struggles to identify antibodies against challenging epitopes. Phage display libraries frequently yield antibodies with suboptimal affinities and poor expression characteristics. Meanwhile, synthetic libraries face the additional limitation of being constrained by design rather than driven by natural immune selection, often lacking the refined specificity and developability characteristics that come from in vivo discovery approaches.
Moreover, maintaining native GPCR conformation throughout the discovery process presents a critical challenge across all traditional platforms. Without proper conformation, these methods often generate antibodies against misfolded or non-physiological receptor forms that fail to recognize the therapeutic target in its native cellular context, resulting in lower affinity candidates that require extensive optimization. These technical barriers have historically made GPCRs among the most challenging targets for therapeutic antibody development.
Next-generation solutions for GPCR targeting
Alloy Therapeutics has developed a fully integrated, high-throughput GPCR antibody discovery platform that breaks through the barriers of traditional approaches. Our ATX-Gx™ transgenic mouse platform features a complete human antibody repertoire with both kappa and lambda light chains, providing the diversity needed to identify high-quality therapeutic candidates. Multiple specialized strain options overcome immunodominance and expand epitopic coverage, enabling robust immune responses, including against high-homology targets.
Successful GPCR antibody discovery requires innovative immunization strategies that preserve native receptor conformation. We utilize genetic-based immunizations, where GPCRs are presented in their native state to maintain proper folding and increase the likelihood of generating antibodies that recognize functional epitopes. Additionally, we encode proprietary molecular adjuvants ensuring a robust epitope-diverse immune response regardless of homology level.
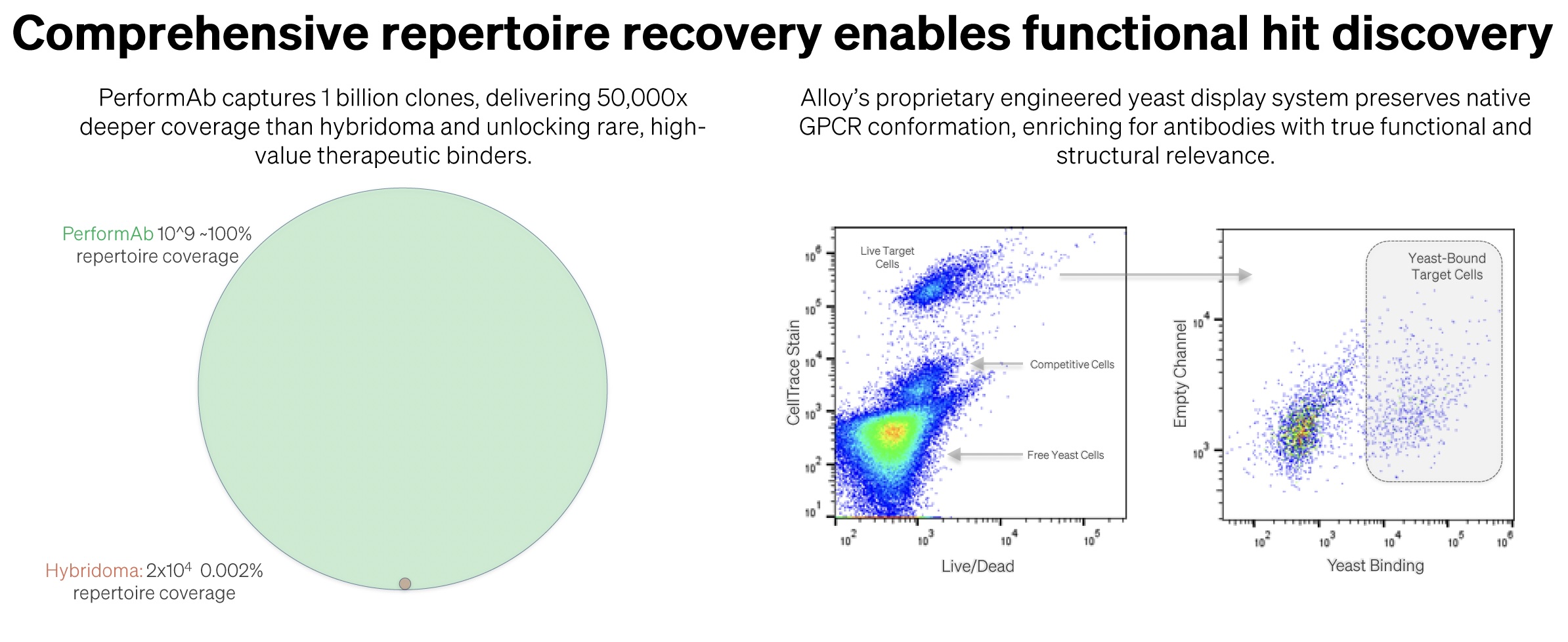
Our PerformAbTM platform also represents a paradigm shift in precision antibody discovery. This advanced yeast display technology overcomes limitations of traditional discovery approaches, for example, hybridoma methods capture only 0.002% of the immune repertoire, while PerformAb captures billions of antibody candidates, achieving nearly 100% repertoire coverage. This exponential increase in sampling depth is complemented by sophisticated screening capabilities.
Our target product profile (TPP)-aligned clone selection process integrates high-throughput screening and sorting to efficiently identify lead candidates. Simultaneously, high-throughput characterization evaluates epitope specificity, species cross-reactivity, and developability profiles, ensuring that only the most promising clones advance. In silico tools flag liabilities early, applying a balanced approach to optimize hit rate without compromising diversity. Candidates with unfavorable profiles are triaged out, accelerating downstream success. These vetted clones then enter our mAbForge™ rapid expression system, where therapeutic relevance and developability characteristics are validated at scale, providing confidence that each lead has the potential to progress toward clinical application.
Case Study: PerformAb success with a difficult-to-target GPCR
A recent antibody discovery program demonstrated the power of the innovative PerformAb platform against a challenging GPCR target. The campaign began with immunizations using ATX-Gx™ kappa light chain and hyperimmune mouse strains, which are optimized for robust immune responses. Within 4-5 weeks, the team observed excellent titers across multiple animals, capturing approximately one billion unique antibody sequences, a 50,000-fold increase over hybridoma methods, establishing a diverse immune repertoire as the foundation for discovery.
The critical challenge came in capturing and enriching this diversity through the transition from mice to yeast display. The team employed sophisticated cell-based screening using GPCR-expressing cells versus knockout controls to eliminate polyreactive antibodies and those binding to non-therapeutic epitopes, enabling precise in vivo repertoire mining to enrich for target-specific binders. Through multiple rounds of sorting and enrichment, robust populations of GPCR-specific binders emerged, differentiated by specificity and developability.
Following enrichment, the GPCR-binding populations underwent sequencing and in silico prioritization. The bioinformatics pipeline systematically removed liability-prone sequences while analyzing both clonotype diversity and heavy-light chain pairings. This balanced approach captured different antibody combinations to maximize repertoire coverage. Leveraging Alloy’s proprietary mAbForge rapid expression system, the team produced 190 monoclonal antibodies in less than 1 week, achieving an average yield of 90μg per clone, ample material for subsequent characterization.
Alloy’s PerformAb platform delivered a large number of functional clones with robust binding profiles. Of the 190 monoclonal antibody candidates produced, 106 demonstrated specific binding to the native GPCR target, an exceptionally high yield for the amount of clonotype diversity assessed. Equally as impressive, the entire workflow was completed in just 12 weeks. This accelerated timeline and high success rate demonstrate how Alloy Therapeutics’ advanced GPCR antibody discovery platform can overcome traditional workflow challenges, expedite delivery of TPP-aligned therapeutic leads, and empower researchers to proceed to functional testing with greater confidence.
End-to-end high-throughput scalable workflow for challenging targets
Download our poster to discover how Alloy’s platforms are applied to accelerate antibody discovery and are able to generate high quality functional leads
Expanding therapeutic possibilities
The availability of high-quality GPCR-targeting antibodies opens new therapeutic possibilities beyond conventional applications. Bispecific antibodies can simultaneously engage GPCRs and other targets, antibody drug conjugates (ADCs) targeting GPCRs enable selective delivery of cytotoxic payloads to specific cell types, while GPCR-targeted cell therapies represent an emerging frontier in immunotherapy. Combination approaches that leverage GPCR-targeted antibodies alongside other therapeutic agents offer opportunities for synergistic effects and improved clinical outcomes.
Advancements in GPCR antibody discovery technologies are making previously unattainable GPCR targets more accessible, proving particularly exciting for addressing complex diseases where other approaches have proven insufficient. From metabolic disorders and cardiovascular disease to neurological conditions and cancer, the ability to generate therapeutic antibodies against challenging GPCRs promises to deliver new treatment options for patients.
Advancing precision therapeutics
The convergence of advanced transgenic humanized mouse platforms, innovative immunization strategies, and high-throughput discovery technologies is finally unlocking the therapeutic potential of GPCR-targeting antibodies. By overcoming the structural and immunological challenges that have historically limited the field, platforms like ATX-Gx™ and PerformAb are enabling the rapid discovery of high-quality therapeutic candidates against challenging GPCR targets. As the field continues to evolve, the result will be the next generation of precision therapeutics.
Ready to tackle your challenging GPCR target? Contact Alloy Therapeutics to learn how our integrated discovery platforms can accelerate your path to breakthrough therapies.